CTMS market to surpass the billion-dollar frontier by 2024, penetration of big data analytics to sway the business trends
Publisher : Fractovia | Published Date : 2018-06-07Request Sample
With healthcare cost and patent cliff reaching new highs, clinical trial management systems (CTMS) market has witnessed a revolutionary transformation over the recent years, primarily in terms of operational model. As per estimates, global spending on medications is forecast to record more than USD 1 trillion by 2022. Almost a decade back, the expenditure was near about USD 885 billion. In the face of this sudden escalation, CTMS industry is apparently approaching new era of trial research that is more streamlined and patient centric. A plethora of off-beat innovations that the business space is presently characterized by can be placed as viable substantiation to the aforementioned fact.
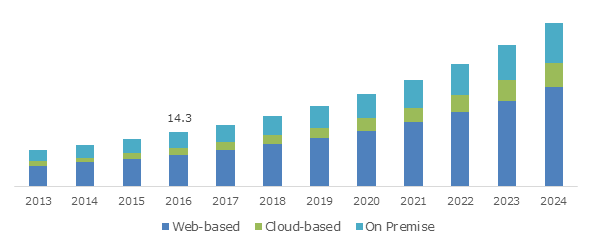
India Clinical Trial Management Systems Market, By Delivery Mode, 2013 - 2024
In this regard, the latest scoop that is grabbing most of the headlines in clinical trial management systems industry is OmniComm Systems’ launch of TrialMaster Version 5. This breakthrough user-friendly solution for electronic data capture is claimed to be exceptionally featured with intuitive user interface, ePRO (electronic patient reported outcomes) capability, risk based monitoring tools and mobile friendly technology. Quite evidently coherent from its credentials, this enhanced version of TrialMaster EDC is likely to be a milestone innovation in CTMS market, making OmniComm Systems’ stance more pronounced in the competitive landscape.
Technological advancements are undeniably catapulting clinical trials into a new age of scientific breakthroughs, where the industry is slated to scale up the productivity spectrum with cost effective outcome-based approach. With huge influx of data in pharma space, the complexity of clinical trial management systems market is increasing. Amidst this backdrop, clinical trial management systems market players, as it is observed lately, have been showing great interest in big data analytics approach, enabling an efficient and fast track data collection, data security, and data management. The affluence of big data analytics is vividly coherent from the estimation that claims cloud based CTMS industry to witness a remarkable CAGR of 15% over 2017-2024.
The advanced methodologies if incorporated into conventional randomized controlled trial (RCT) model are deemed to tackle a slew of limitations such as reproducibility, infrequent external validation, generalizability, and cost issues. Not to mention, with connected technologies such as machine learning, artificial intelligence, and cloud computing becoming mainstream, clinical trial management systems industry is set to witness a disruption of sorts over the ensuing years.
Yet another trend that is expected to leave an inexorable impact on CTMS marketplace is outsourcing of clinical research. As pharma players are constantly combating with the pricing pressure, outsourcing has turned out to be a smarter and innovative strategy. A multitude of factors are responsible for this growing dependence on clinical research outsourcing, capacity constraints being at the pinnacle. Reportedly, since 2007 renowned pharmaceutical behemoths including Merck, Pfizer, and AstraZeneca have underlined plans to downsize and consolidate their operations. While as of now CROs are offering preclinical as well as clinical services, they are soon expected to widen their spectrum into trial management projects.
It is imperative to mention that strategic collaborations have been one of the top-notch strategy implemented by the clinical trial management systems industry players, with an intent to strengthen their business position. Adventist Health System’s yesteryear partnership with Bio-Optronics is an apt instance depicting the same. Reportedly, the agreement encompassed initial roll out of clinical conductor CTMS at two of the eminent research institutes of AHS. The deployment included the integration of Cerner Millennium with CTMS-EMR, which led to an improved research quality and process efficiency. The deal enabled AHS to deliver streamlined clinical trials that served the patient requirement as well as led to myriad advancements in medical research. In essence, these appreciable efforts by the market giants are sure to bear fruit in the years ahead. Speaking of commercialization, Global Market Insights, Inc., forecasts the overall clinical trial management systems industry to exceed a valuation of USD 2.4 billion by 2024.