ENT (Ear, Nose, Throat) devices market to derive extensive receipts via hearing aids product sales over 2017-2024, massive R&D activities to characterize industry landscape
Publisher : Fractovia | Published Date : 2018-06-08Request Sample
Predominantly driven by the rising incidences of hearing loss, throat infections, and nasal ailments, the global ENT (Ear, Nose, Throat) devices market share has been witnessing an enormous upsurge over the last decade. Major firms partaking in this business sphere have been deploying their resources toward developing enhanced surgical systems and are increasingly focusing on technological advancements in hearing aids – a factor that is slated to augment the growth prospects of ENT devices industry. In fact, as per reliable estimates, the overall ENT devices market amassed a total remuneration of about USD 13.4 billion in 2016, which underlines the lucrative growth opportunities awaiting the stakeholders of this business space.
UK ENT (Ear, Nose, Throat) Market, By Product, 2013 – 2024 (USD Million)
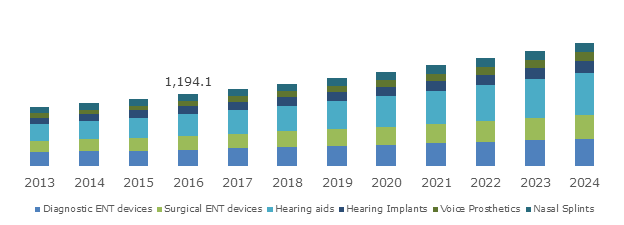
Hearing aids segment to emerge as the forerunner in the product landscape of ENT devices industry
The unprecedented increase in hearing loss, specifically among the geriatric populace, has been the pivotal cause to have impelled the hearing aids ENT devices industry share. In fact, according to a research report by Global Market Insights, Inc., hearing aids segment apportioned the largest revenue share of the overall business sphere in the year 2016.
Add to it, a marked increase in government-initiated awareness programs across various developing nations has further boosted the ENT devices market trends. For instance, the Social Justice and Empowerment Ministry of India, through its Department of Empowerment of Persons with Disabilities (DEPwD), had recently organized a ‘Cochlear Implant Awareness Program’ in the state of Haryana. Reportedly, the event, named as Swar Swagtam, aimed at creating awareness among the masses about the government’s Cochlear Implant Program under the Assistance to Disabled persons (ADIP) scheme of the ministry.
Apparently, the aforementioned instance goes on to underline the efforts being undertaken by several countries to assist the economically weaker section of the society to avail the benefits of modern technology such as Cochlear Implants which are highly expensive. Needless to mention, such initiatives would massively boost the hearing aids product sales in the times to come.
Growing emphasis on research and development programs to have an optimistic impact on ENT devices industry
Concurrently, it has been observed that numerous prominent hearing aid makers are increasingly reshaping their business strategies by stressing on research and development (R&D) activities to enhance their existing product range. For instance, one of the world’s foremost hearing aid manufacturers, Widex, has recently announced to merge with Singapore-headquartered Sivantos in a bid constitute a new company that would exclusively focus on R&D programs. If reports are to be believed, both the firms would invest massively to form the company which is anticipated to focus on enhancing hearing aid features by equipping them with advanced sensors and Bluetooth.
The soon-to-be-established firm, which is said to be the third largest in the hearing aids segment of the global ENT devices market with an astonishing valuation of more than USD 8.3 billion, would enable Widex and Sivantos to swiftly adapt to the demands of digital age and a more tech-savvy population. Moreover, the R&D activities would aid both the firms in experimenting with new approaches and challenge the conventional model of selling hearing aids through specialist retailers.
Citing a yet another instance that highlights the significance of R&D programs in transforming the growth landscape of ENT devices industry, Cochlear Limited announced toward the end of 2017 that the U.S. Food and Drug Administration (FDA) has approved its new remote feature. Apparently, the feature has been designed for the Nucleus Cochlear Implant System of the Australia based medical devices giant. It would reportedly enable healthcare specialists to perform follow-up programming on a patient’s cochlear implant remotely via a telemedicine platform.
Moreover, it is quite imperative to state that the U.S. FDA has termed the latest feature, which has been primarily targeted toward the patients who have had at least six months of experience with their implant sound processor, to be highly promising as it can drastically enrich the user’s quality of life. The said feature reportedly enhances the user’s ability to comprehend speech or music and serves as a substantial relief to certain hearing loss patients.
Taking into account the aforementioned instances, the overall business space is anticipated to benefit from the increasing research activities and pioneering technological advancements over the next few years. Driven by the rising number of awareness programs across several emerging nations and increasing accessibility of high-grade products, the ENT devices market is slated to surpass a stupendous revenue collection of around USD 24.5 billion by 2024.