In-vitro diagnostic services market to garner lucrative proceeds from software services over 2018-2024, supportive government initiatives to augment the industry landscape
Publisher : Fractovia | Published Date : 2018-10-05Request Sample
With rapid developments in diagnostic testing technology, the global in-vitro diagnostic services market, over the recent years has significantly attracted the interest of investors and major healthcare providers. In fact, according to statistics, diagnosis and clinical testing is used in more than 70% of healthcare decisions and can help provide targeted therapy at much lower costs. The global in-vitro diagnostic services market, in this regard is making a strong headway in the healthcare sector and was worth USD 44 billion in 2017.
U.S. In-vitro Diagnostic Services Market, By Software, 2013 – 2024 (USD Billion)
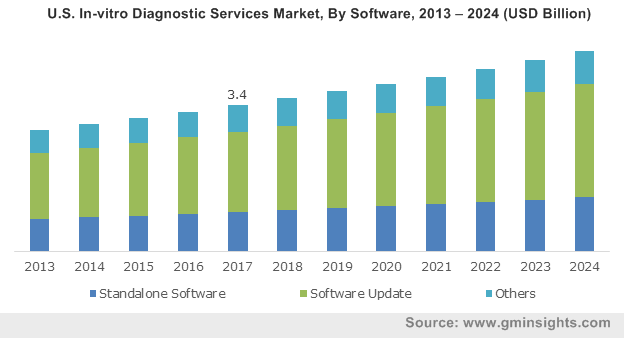
Elaborating further, the robust expansion of this business sphere can be chiefly attributed to the growing prevalence of chronic diseases and the overall developments in diagnostics and healthcare infrastructure. As per the statistics provided by the World Health Organization, in 2015, over 24.6 million people worldwide suffered from cancer and globally there were more than 8.8 million deaths due to cancer. Add to it, the rising incidences of colorectal cancer, which according to the World Cancer Research Fund International recorded 1.4 million new cases in 2018. Quite overtly, this increasing rate of cancer and the pickup in demand for effective cancer diagnostics have positively impacted the growth of the in-vitro diagnostic services market in the recent years.
Software in-vitro diagnostic services market to emerge as lucrative investment spot over 2018-2024
Of the two in-vitro diagnostic services, which includes software and testing services, the software segment is gaining immense traction lately. If industry analysts are to be believed, the software diagnostic services are favorably driving the digital transformation in the healthcare industry and are therefore being adopted by several hospitals and point-of-care testing centers. These software services moreover are playing an increasingly significant role in the drug discovery processes via gene sequencing analysis, which is why this segment is expected to be a potential investment ground. Estimates claim the global software in-vitro diagnostic services market to record a CAGR of 4.5% over 2018-2024.
Analyzing the massive growth potential, it is prudent to mention that several prominent industry behemoths are adopting market growth strategies such as M&As, partnerships, JVs, and R&D to expand their business in software diagnostics services. Recently for instance, Roche Diagnostics, partnered with tech-giants such as Microsoft and Cleidon to expand software offering services for providing better healthcare solutions as well as for global business expansion.
How industrial and governmental backing is influencing the commercialization potential of in-vitro diagnostic services market?
Recently, in June 2018, NAMSA, the world’s only Medical Research Organization (MRO) that helps accelerate the development of medical devices through integrated clinical research, laboratory testing, and regulatory consulting services has successfully launched its in-vitro diagnostics development business. This platform, according to reports is expected to provide the global manufacturers a proven resource for commercializing the outcomes delivered via NAMSA’s clinical research services.
These regulatory and clinical services have grabbed the attention of several healthcare providers for a more rapid treatment and diagnosis plans, in turn intensifying in-vitro diagnostic services market demand. Reports cite that the sponsors, who have limited options for IVD-focused CRO (Contract Research Organizations) will benefit majorly from these new service offerings.
In yet another recent instance fortifying the commercial growth of in-vitro diagnostic services industry, OptraSCAN, a leading digital pathology solution provider, has announced that it has received CE mark approval for its whole-slide scanners used in in-vitro diagnostic services. Quite overtly, the CE mark has opened the gates for OptraSCAN’s scanners to be sold in countries under or affiliated to the European Union, in turn driving in-vitro diagnostic services industry share.
In line with such increasing support from the competitive landscape and with the advent of new technologies such as big data analytics, cloud computing, and Internet of Things, the in-vitro diagnostic services market has emerged as a profitable business sphere of the healthcare industry. All in all, the rising prevalence of cancer and other chronic disease and the supportive competitive scenario is further likely to enrich the growth dynamics of global in-vitro diagnostic services industry in the coming years. The global in-vitro diagnostic services market is forecast to cross USD 63 billion by 2024, as compiled by Global Market Insights, Inc.