U.S. pharmacovigilance market to amass substantial returns over 2017-2024, contract outsourcing to emerge as the leading service provider segment
Publisher : Fractovia | Published Date : 2017-04-13Request Sample
The significance of mandatory drug safety monitoring has had a critical impact on pharmacovigilance market, that has been gaining remarkable momentum lately. Driven by a strict regulatory framework pertaining to drug testing and approval, leading pharmacovigilance service providers often collaborate with CROs to maximize their service portfolio and offer customized, foolproof services. Additionally, even major geographies have been on the run to modernize their pharmacovigilance service portfolios in order to consolidate their stance in the regional landscape of pharmacovigilance industry.
An instance validating the authenticity of the aforementioned fact is that of Indian pharmaceutical space looking forward to incorporating big data analytics in the pharmacovigilance market. The intention behind the strategy is to simplify the huge volume of information, structured and unstructured, using simple data base management. Pharmacovigilance services are now being outsourced to big-shot companies such as Accenture, Cognizant Technology Solutions Corporation, Quintiles, Boehringer Ingelheim, Synowlwedge, Bristol-Myers Squibb, ICON, United BioSource, PAREXEL, and Covance. Unique services ranging from drug testing, drug approval, and drug commercialization provided by these pharmacovigilance industry players are likely to augment the revenue graph of this vertical. A report by Global Market Insights, Inc., states that pharmacovigilance market size stood at more than USD 3.2 billion in 2016.
India Pharmacovigilance Market, By Clinical Trial Phase, 2012 - 2024
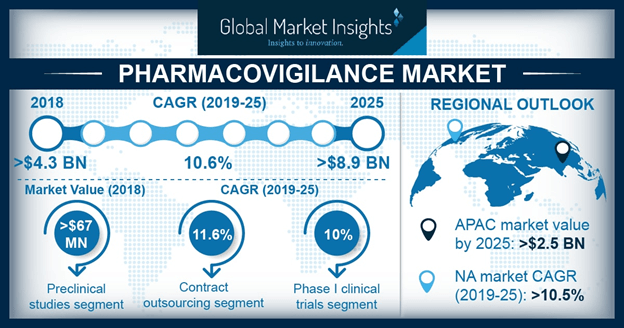
The pharmaceutical sector is on an inclined growth path with the rising number of global chronic disorders and the subsequent increase in the manufacture of suitable drugs. In 2010, more than 6 billion drugs were disbursed in the United States alone. Manufactured drugs, quite overtly, are clinically tested for adverse effects and fatal reactions, prior to being commercialized and made available to the masses. With public health and safety being prioritized here, the pharma space is naturally fueled by strict norms. By extension, it would be fair to state that stringent government regulations, many of which have been upgraded and revised, subject to clinical trials and drug approvals, will undeniably drive global pharmacovigilance industry trends in the forthcoming years.
Pharmacovigilance services, though nowadays are outsourced on a contractual basis, are offered in-house as well, pertaining to the customized demand by several companies. The request plausibly comes on the heels of the necessity to maintain data confidentiality and security in a high-risk environment. However, contract outsourcing services have emerged as a go-to option for many companies, as contract research organizations are renowned to provide specialized services and ensure accurate monitoring of the effects of the drugs at a much lower cost. In accordance, numerous manufacturers are now outsourcing their pharmacovigilance related work to pivotal companies, specifically in the emerging economies.
Speaking along similar lines, it would be rather overt to state that contract outsourcing has provided a way for nations across the APAC to upsurge the regional pharmacovigilance market. China for instance, has been fast emerging as a crucial revenue contributor for APAC, given the expanding pharma space in the country and the increasing number of favorable government policies related to drug safety. Japan in fact, dominated APAC pharmacovigilance market on account of robust healthcare infrastructure and an excellent adverse drug reaction reporting system.
Currently, even pharmaceutical and biotechnology firms are concentrating on the bottom line range and outsourcing pharmacovigilance services to niche-specific organizations. Outsourcing these services will reduce the financial losses pertaining to testing failures and drug approval delays as well, which is sure to act as a major driving force for pharmacovigilance industry share from contract outsourcing. Indeed, as per analysts, contract outsourcing emerged as the dominant service provider segment in 2016 and is anticipated to continue its dominance in pharmacovigilance industry over 2017-2024.
The United States has been forecast to come up as one of the most remunerative regions for the growth of pharmacovigilance market. Indeed, U.S. led North America pharmacovigilance market in 2016, driven by the highly supportive regulatory framework in the country. The regulations have been duly framed in accordance with the post marketing surveillance as well as the rising concerns related to drug safety.
One of the primary reasons for pharmacovigilance industry outlook to have observed a change in the United States in recent times is the myriad number of deaths in the country associated with adverse drug reactions. This in consequence led to an upscale in the demand for enhanced drug monitoring & reporting services, that is likely to propel U.S. pharmacovigilance market.
Pharmacovigilance market participants in the developed regions have registered a paradigm shift in the provision of their services over the last few years. Most of the leading providers are now outsourcing their services to the BPOs and other related firms in the emerging economies, owing to the fact outsourcing reduces operational costs, aids safer drug monitoring, ensures adherence to regulatory reforms, and helps enhance the overall productivity. This in consequence, will stimulate global pharmacovigilance industry size, slated to cross USD 8 billion by 2024.